
Kamal Medtech is into the medical device industry such as Dental Implantology & Intervention Cardiology
Kamal Medtech is into the medical device industry such as Dental Implantology & Intervention Cardiology and has a wide distribution network through its direct sales team present across India. Kamal Medtech has signed an exclusive distribution agreement with Medbone Biomaterials to distribute their dental product portfolio in India.


Medbone was founded in 2008, with the purpose to fill a gap in the market in the area of synthetic bone graft manufacturing. Medbone is committed to meet customer requirements, in order to enhance their satisfaction. This commitment can be seen through the continuous improvement of our products and services, as well as, through the continuous upgrade in our Quality Management System based on ISO 13485 and the standards of Risk Management System based on ISO 14971.
There are wide applications of Biomaterials worldwide, being used in more than 90 countries, in orthopedic, dental, and veterinary surgeries. Medbone is constantly expanding the range of applications, in order to respond to the growing needs of health professionals through the development of new medical devices
Medbone products have synthetic origin, which has major advantages compared to other solutions on the market: there is no risk of infections, no contraindications, and all of our products are 100% resorbable, mimicking natural bone.
Medbone wants to meet the needs of the market. For the same reason, the company every day is working towards the development of new products and with increasingly diversified applications.




0.1 – 0.5 mm

0.5 – 1 mm

1 – 2 mm


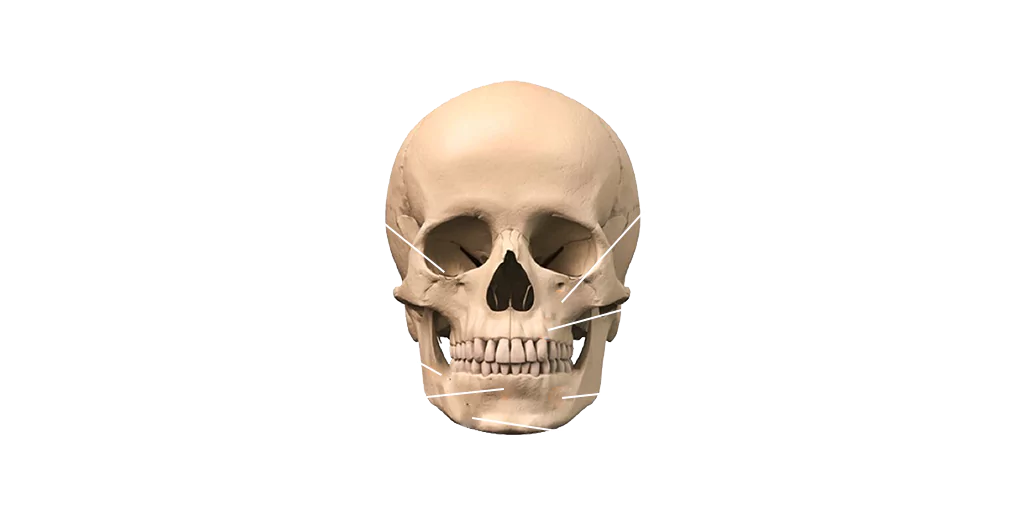
adbone TCP can be easily mixed with patient’s blood. The hydrophilic behavior of adbone TCP confers a high cohesivity of the particles
adbone TCP does not contain animal or human tissues or derivatives
The interconnected porosity of adbone TCP forms an idal environment for vascularization
adbone TCP is radiopaque, allowing the monitorization of the graft osteointegration
The use of membrane is not required unless there is risk of graft exposure



| References | Geometry | Range Size | Quantity |
|---|---|---|---|
| TCP010505G | Granules | 0.1 – 0.5 mm | 0.5g x 1 Unit |
| TCP050105G | Granules | 0.5 – 1 mm | 0.5g x 1 Unit |
| TCP010505P | Granules | 0.1 – 0.5 mm | 0.5g x 5 Unit |
| TCP050105P | Granules | 0.5 – 1 mm | 0.5g x 5 Unit |
| TCP010510G | Granules | 0.5 – 1 mm | 1g x 1 Unit |
| TCP050110G | Granules | 0.5 – 1 mm | 0.5g x 5 Unit |
| TCP010210G | Granules | 1 – 2 mm | 1g x 1 Unit |
| TCP010510P | Granules | 0.1 – 0.5 mm | 1g x 5 Unit |
| TCP050110P | Granules | 0.5 – 1 mm | 1g x 5 Unit |
| TCP010210G | Granules | 1 – 2 mm | 1g x 5 Unit |
| References | Geometry | Range Size | Quantity |
|---|---|---|---|
| TCP051015B | Blocks | 5 x 10 x 15 mm | 1 Unit |
| TCP010505G | Blocks | 8 x 8 x 20 mm | 1 Unit |
| TCP010505G | Blocks | 15 x 15 x 20 mm | 1 Unit |
| References | Geometry | Range Size | Quantity |
|---|---|---|---|
| TCP010505G | Blocks | 5 x 10 x 15 mm | 1 Unit |
| TCP010505G | Blocks | 8 x 8 x 20 mm | 1 Unit |
| TCP010505G | Blocks | 15 x 15 x 20 mm | 1 Unit |
| References | Geometry | Range Size | Quantity |
|---|---|---|---|
| BCP010510P | Granules | 0.1 – 0.5 mm | 1g x 5 Units |
| BCP050110P | Granules | 0.5 – 1 mm | 1g x 5 Units |
| BCP010210P | Granules | 1 – 2 mm | 1g x 5 Units |
| BCP030405G | Granules | 3 – 4 mm | 5g x 1 Unit |
| BCP030410G | Granules | 3 – 4 mm | 10g x 1 Unit |
| BCP030415G | Granules | 3 – 4 mm | 15g x 1 Unit |
| BCP030420G | Granules | 3 – 4 mm | 20g x 1 Unit |
| BCP030430G | Granules | 3 – 4 mm | 30g x 1 Unit |
| BCP040705G | Crunch | 4 – 7 mm | 5g x 1 Unit |
| BCP040710G | Crunch | 4 – 7 mm | 10g x 1 Unit |
| BCP040715G | Crunch | 4 – 7 mm | 15g x 1 Unit |
| BCP040710G | Crunch | 4 – 7 mm | 20g x 1 Unit |
| BCP040720G | Crunch | 4 – 7 mm | 30g x 1 Unit |
| References | Geometry | Range Size | Quantity |
|---|---|---|---|
| BCP080820B | Block | 8 x 8 x 20 mm | 1 Unit |
| BCP151520B | Block | 15 x 15 x 20 mm | 1 Unit |
| BCP152030B | Block | 15 x 20 x 30 mm | 1 Unit |
| BCP080820C | Cylinder | 8 x 8 x 20 mm | 1 Unit |
| BCP040420F | Stick | 4 x 4 x 20 mm | 5 Units |
| BCP040420Q | Stick | 4 x 4 x 20 mm | 4 Units |
| References | Geometry | Range Size | Quantity |
|---|---|---|---|
| BCP062530W | Wedge | 6 x 25 x 30 mm | 1 Unit |
| BCP082530W | Wedge | 8 x 25 x 30 mm | 1 Unit |
| BCP102530W | Wedge | 10 x 25 x 30 mm | 1 Unit |
| BCP122530W | Wedge | 12 x 25 x 30 mm | 1 Unit |
| BCP142530W | Wedge | 14 x 25 x 30 mm | 1 Unit |