
Trackmaster, the next-generation Everolimus-Eluting Coronary Stent System, is designed for exceptional flexibility, controlled drug release, and superior clinical outcomes. Engineered with ultra-thin strut technology, Trackmaster ensures seamless vessel adaptability, reduced arterial injury, and optimized endothelialization.
Manufactured at Kamal Medtech’s state-of-the-art 50,000 sq. ft. facility, Trackmaster meets global healthcare standards, offering unmatched safety and effectiveness for complex coronary interventions.

Controlled Everolimus release up to 50 days, ensuring gradual healing and minimal restenosis risk.
Fully degrades through hydrolysis and enzymatic degradation, naturally excreting from the body as CO₂ and H₂O.
Enhances stent flexibility, reducing vessel trauma and improving crossability in complex anatomies.
Ensures uniform vessel support, maintaining integrity without foreshortening or recoil.
Designed for superior deliverability in tortuous vessels, offering seamless tracking and expansion.
Biodegradable polymers blend, act as a binding factor for the drug. The unique and validated formulation help drug release in a controlled fashion.


Meticulously laser cut Cobalt Chromium Stent proves to be an ideal platform for DES, contributing to high flexibility, conformability, unidirectional expansion with no edge flaring and minimum recoil.
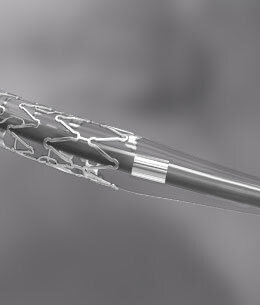
The cytostatic drug, inhibits blood vessel restenosis by limiting the proliferation of smooth muscle cells
| Design: | Open Cell Stent Design | ||
|---|---|---|---|
| Material: | Cobalt Chromium (CoCr) L605 | ||
| Length (mm): | 8, 13, 16, 20, 24, 28, 32, 36, 40, 43 8 47 | ||
| Diameter (mm): | 2.00, 2.25, 2.50, 2.75, 3.00, 3.50, 4.00 & 4.50 | ||
| Strut Dimensions: | Thickness 60-65 um | Strut 70 µm | Connectors 50 µm | ||
| Nominal Pressure (NP): | 9 atm | ||
| Rated Burst Pressure (RBP): | 16 atm | ||
| Foreshortening: | Nearly Zero | ||
| Recoil: | <_5% | ||
| Crossing Profile: | Nearly 100 mm | ||
| Min. Guidewire Diameter: | 0.014” | ||
| Min. Guiding Catheter I.D.: | 5 Fr Compatible | ||
| Radial Strength: | Excellent | ||
| Flexibility: | Excellent | ||
| Dia(mm) | 8 | 13 | 16 | 20 | 24 | 28 | 32 | 36 | 40 | 43 | 47 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.00 | TR20008 | TR20013 | TR20016 | TR20020 | TR20024 | TR20028 | TR20032 | TR20036 | TR20040 | TR20043 | TR20047 |
| 225 | TR22508 | TR22513 | TR22516 | TR22520 | TR22524 | TR22528 | TR22532 | TR22536 | TR22540 | TR22543 | TR22547 |
| 250 | TR25008 | TR25013 | TR25016 | TR25020 | TR25024 | TR25028 | TR25032 | TR25036 | TR25040 | TR25043 | TR25047 |
| 2.75 | TR27508 | TR27513 | TR27516 | TR27520 | TR27524 | TR27528 | TR27532 | TR27536 | TR27540 | TR27543 | TR27547 |
| 3.00 | TR30008 | TR30013 | TR30016 | TR30020 | TR30024 | TR30028 | TR30032 | TR30036 | TR30040 | TR30043 | TR30047 |
| 350 | TR35008 | TR35013 | TR35016 | TR35020 | TR35024 | TR35028 | TR35032 | TR35036 | TR35040 | TR35043 | TR35047 |
| 4.00 | TR40008 | TR40013 | TR40016 | TR40020 | TR40024 | TR40028 | TR40032 | TR40036 | TR40040 | TR40043 | TR40047 |
| 450 | TR45008 | TR45013 | TR45016 | TR45020 | TR45024 | TR45028 | TR45032 | TR45036 | TR45040 | TR45043 | TR45047 |